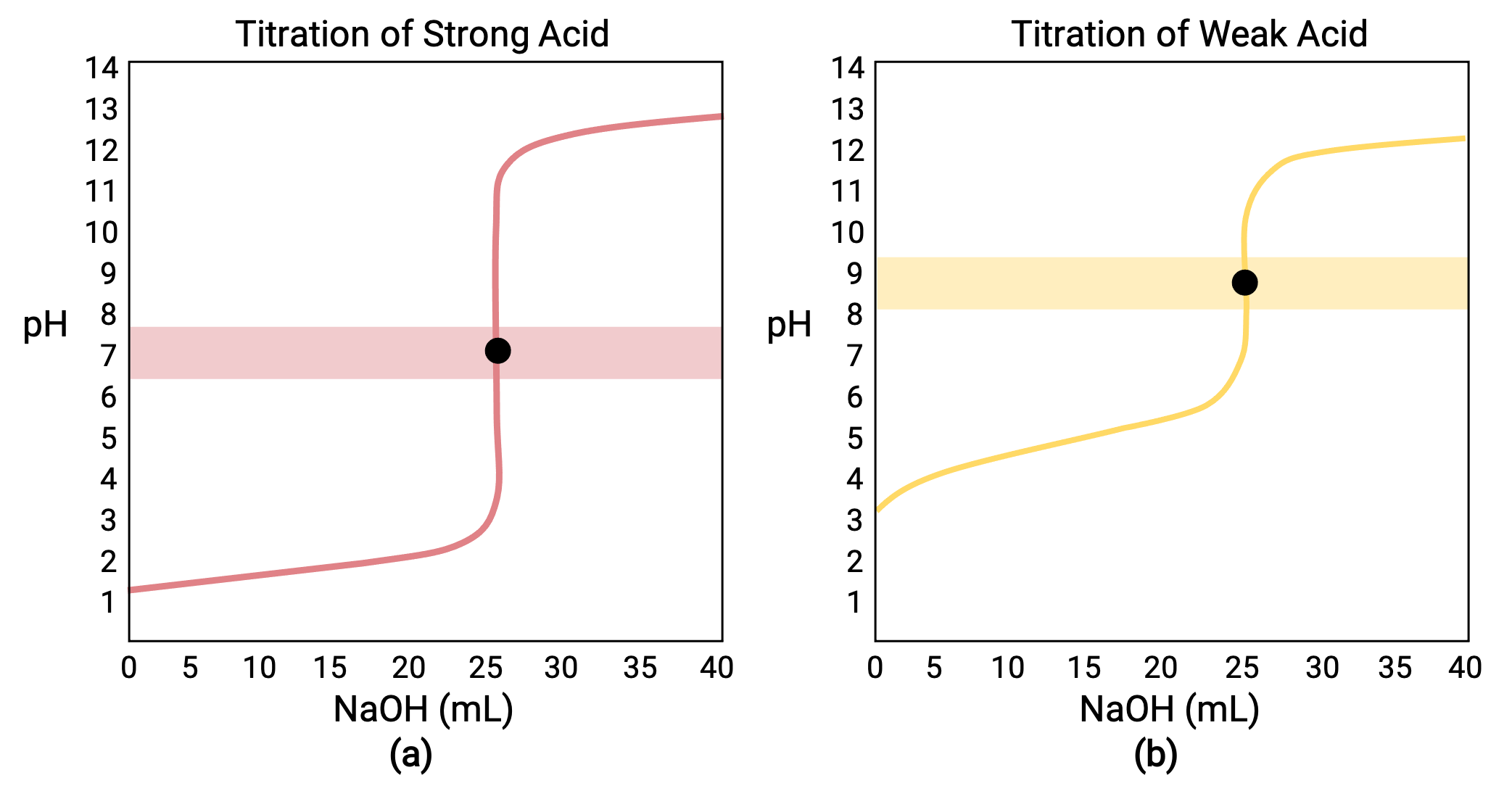
Titration Point Vs Equivalence Point . Point) is the exact point at which the chemical reaction in a titration mixture. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. the equivalence point (eq. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. Endpoint and equivalence point are two terms commonly used in titration experiments. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. The term equivalence point means that the solutions have been mixed. The term neutral point is best avoided.
from exoaqbcwf.blob.core.windows.net
Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. the equivalence point (eq. The term equivalence point means that the solutions have been mixed. Point) is the exact point at which the chemical reaction in a titration mixture. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The term neutral point is best avoided. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample.
Equivalence Point Titration With Base at Alvin Barajas blog
Titration Point Vs Equivalence Point the equivalence point (eq. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. Point) is the exact point at which the chemical reaction in a titration mixture. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. Endpoint and equivalence point are two terms commonly used in titration experiments. the equivalence point (eq. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there Titration Point Vs Equivalence Point the equivalence point (eq. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed. Point) is the exact point at which the chemical reaction in a titration mixture. a point of equivalence. Titration Point Vs Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Point Vs Equivalence Point a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Point) is the exact point at which the chemical reaction in a titration mixture. The term equivalence point means that the solutions have been mixed. the equivalence point (eq. Endpoint and equivalence point. Titration Point Vs Equivalence Point.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a Titration Point Vs Equivalence Point a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. The term equivalence point means that the solutions have been mixed. The term neutral point is best avoided. Endpoint and equivalence point are two terms commonly used in titration experiments. the equivalence point (eq. In a. Titration Point Vs Equivalence Point.
From mavink.com
Titration Endpoint Vs Equivalence Point Titration Point Vs Equivalence Point the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. Endpoint and equivalence point are two terms commonly used in titration experiments. the equivalence point (eq. The term neutral point is best avoided. a titration is a volumetric technique in which a. Titration Point Vs Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Point Vs Equivalence Point a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. Endpoint and equivalence point are two terms commonly used in titration experiments. Point) is the exact point at which the chemical reaction in a titration mixture. the equivalence point or stoichiometric point is the point in. Titration Point Vs Equivalence Point.
From www.vrogue.co
How To Calculate Pka From The Half Equivalence Point vrogue.co Titration Point Vs Equivalence Point Endpoint and equivalence point are two terms commonly used in titration experiments. The term neutral point is best avoided. the equivalence point (eq. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The term equivalence point means that the solutions have been. Titration Point Vs Equivalence Point.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Point Vs Equivalence Point Endpoint and equivalence point are two terms commonly used in titration experiments. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. the equivalence point (eq. The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed. Point) is the exact point at which. Titration Point Vs Equivalence Point.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Point Vs Equivalence Point In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. The term equivalence point means that the solutions have been mixed. the equivalence point (eq. The term neutral point is best avoided. Point) is the exact point at which the chemical reaction in a titration mixture. a titration is a. Titration Point Vs Equivalence Point.
From schoolbag.info
Titration and Buffers Acids and Bases Titration Point Vs Equivalence Point Point) is the exact point at which the chemical reaction in a titration mixture. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent. Titration Point Vs Equivalence Point.
From www.youtube.com
Using the incorrect indicator, acid base titration end point vs Titration Point Vs Equivalence Point Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the. Titration Point Vs Equivalence Point.
From www.youtube.com
Difference Between End Point and Equivalence Point YouTube Titration Point Vs Equivalence Point a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The term neutral point is best avoided. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. the equivalence point. Titration Point Vs Equivalence Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Point Vs Equivalence Point a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The term equivalence point means that the solutions have been mixed. Point) is the exact point at which the chemical reaction in a titration mixture. The term neutral point is best avoided. the. Titration Point Vs Equivalence Point.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Point Vs Equivalence Point The term equivalence point means that the solutions have been mixed. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. Endpoint and equivalence point are two terms commonly used in titration experiments. the equivalence point (eq. a titration is a volumetric technique in which. Titration Point Vs Equivalence Point.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Titration Point Vs Equivalence Point Endpoint and equivalence point are two terms commonly used in titration experiments. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. the equivalence point or stoichiometric point. Titration Point Vs Equivalence Point.
From mungfali.com
Half Equivalence Point On Titration Curve Titration Point Vs Equivalence Point the equivalence point (eq. The term equivalence point means that the solutions have been mixed. The term neutral point is best avoided. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. In a titration, it is where the moles of titrant equal. Titration Point Vs Equivalence Point.
From exoaqbcwf.blob.core.windows.net
Equivalence Point Titration With Base at Alvin Barajas blog Titration Point Vs Equivalence Point The term neutral point is best avoided. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Point) is the exact point at which the chemical reaction in a titration mixture. Endpoint and equivalence point are two terms commonly used in titration experiments. . Titration Point Vs Equivalence Point.
From dxomvqiez.blob.core.windows.net
Equivalence Point For Titration Of Strong Acid at Ora Peach blog Titration Point Vs Equivalence Point Point) is the exact point at which the chemical reaction in a titration mixture. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. Endpoint and equivalence point are two terms commonly used in titration experiments. The term neutral point is best avoided. a titration is a volumetric technique in which. Titration Point Vs Equivalence Point.
From mavink.com
Titration Endpoint Vs Equivalence Point Titration Point Vs Equivalence Point The term neutral point is best avoided. Endpoint and equivalence point are two terms commonly used in titration experiments. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to. Titration Point Vs Equivalence Point.